Have you ever wondered how a simple solution like lemon juice can dissolve a hard-boiled egg, or how baking soda can neutralize a spilled acid? This phenomenon lies at the heart of chemistry, governed by the intricate dance of acids and bases.

Image: daotaodaynghe.edu.vn
Understanding acids and bases is crucial for anyone pursuing a career in chemistry, biology, or medicine. It’s the foundation for comprehending chemical reactions, predicting pH levels, and even mastering the art of baking. While the concept of acids and bases might seem intimidating at first, it becomes manageable with practice and a clear understanding of the key principles. This article aims to equip you with the tools and resources to tackle acids and bases calculations confidently, making the seemingly complex world of chemistry a little less daunting.
The Fundamental Concepts: Defining Acids and Bases
Let’s embark on our journey by first defining our key players: acids and bases. Acids, often recognized by their sour taste, possess the ability to donate protons (H+) in a solution. Think of lemons, vinegar, and even your stomach’s digestive juices – these all contain acidic compounds. In contrast, we have bases, which are known for their bitter taste and ability to accept protons (H+). Examples of bases include baking soda, ammonia, and even soap.
Understanding pH: The Acidity or Basicity Scale
The acidity or basicity of a solution is quantified by the pH scale, which ranges from 0 to 14. A pH of 0 indicates a highly acidic solution, while a pH of 14 signifies a highly basic solution. A neutral solution, like pure water, has a pH of 7. This scale provides a convenient way to measure the concentration of H+ ions, with lower pH values representing a higher concentration of H+ ions.
Essential Equations: The Foundation of Acid-Base Calculations
To successfully navigate the world of acid-base calculations, we need to familiarize ourselves with some fundamental equations:
- Concentration (Molarity): Molarity (M) represents the concentration of a solution expressed as moles of solute per liter of solution. It is calculated using the formula: M = moles of solute / liters of solution.
- pH and pOH: The pH scale is directly related to the concentration of H+ ions, and the pOH scale is associated with the concentration of OH- ions. They are calculated using these equations:
- pH = -log[H+]
- pOH = -log[OH-]
- The Relationship between pH and pOH: The pH and pOH values are intrinsically related by the following equation: pH + pOH = 14.
- Ionization Constant (Ka and Kb): These constants play a vital role in quantifying the strength of an acid or base. They indicate the degree to which an acid or base ionizes in solution.
- Ka = [H+][A-] / [HA] for a weak acid
- Kb = [BH+][OH-] / [B] for a weak base
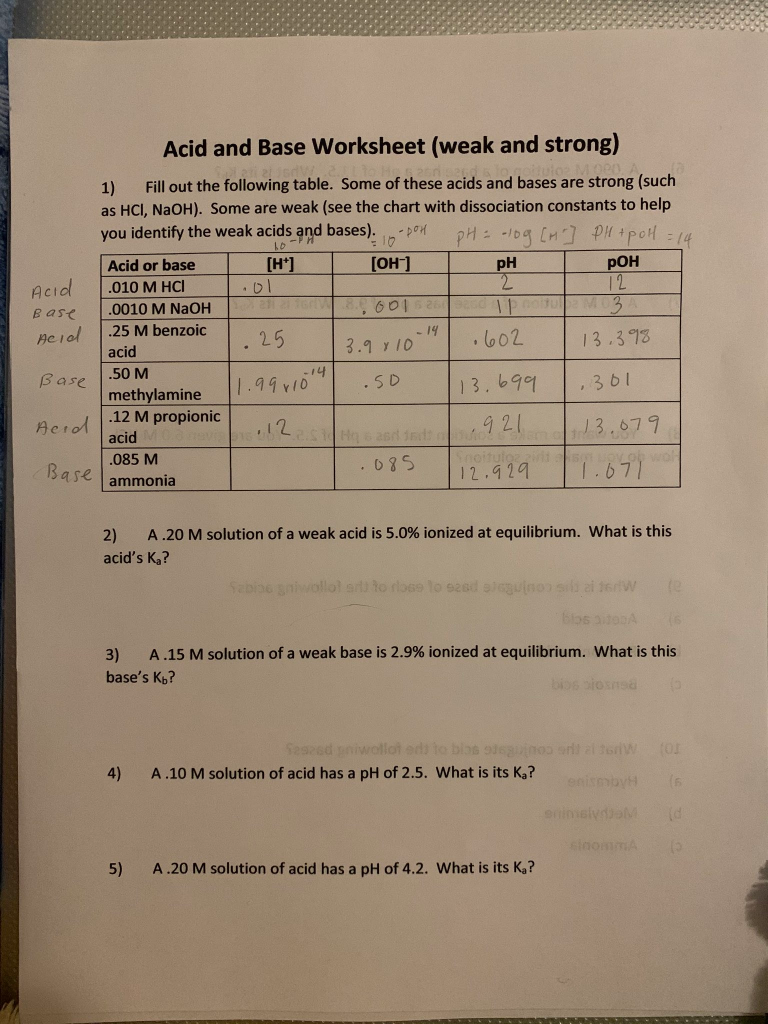
Image: www.e-streetlight.com
Practical Examples: Solving Acid-Base Calculations
Let’s move from theory to practice by exploring a few examples:
Example 1: Calculating pH from Molarity
What is the pH of a 0.1 M hydrochloric acid (HCl) solution?
HCl is a strong acid, meaning it completely ionizes in solution. Therefore, the concentration of H+ ions is equal to the concentration of HCl: [H+] = 0.1 M.
Now, using the equation pH = -log[H+], we can calculate the pH:
pH = -log(0.1) = 1.
Hence, the pH of a 0.1 M HCl solution is 1, making it highly acidic.
Example 2: Calculating the pOH of a Basic Solution
What is the pOH of a 0.05 M sodium hydroxide (NaOH) solution?
NaOH is a strong base, and it fully ionizes in solution, making the concentration of OH- ions equal to the concentration of NaOH: [OH-] = 0.05 M.
Using the equation pOH = -log[OH-], we get:
pOH = -log(0.05) = 1.3.
Therefore, the pOH of a 0.05 M NaOH solution is 1.3.
Example 3: Calculating the pH of a weak acid
Acetic acid (CH3COOH) is a weak acid with a Ka value of 1.8 x 10^-5. Calculate the pH of a 0.1 M acetic acid solution.
To solve this, we need to use the equilibrium expression for the ionization of acetic acid:
CH3COOH(aq) ↔ H+(aq) + CH3COO-(aq)
We set up an ICE table (Initial, Change, Equilibrium) to determine the equilibrium concentrations of all species:
| Species | Initial (I) | Change (C) | Equilibrium (E) |
|---|---|---|---|
| CH3COOH | 0.1 M | -x | 0.1-x |
| H+ | 0 | +x | x |
| CH3COO- | 0 | +x | x |
Substituting the equilibrium concentrations into the Ka expression:
Ka = (x)(x) / (0.1-x) = 1.8 x 10^-5
Since Ka is very small, we can simplify the equation by assuming x << 0.1:
1.8 x 10^-5 ≈ x^2 / 0.1
Solving for x:
x = √(1.8 x 10^-5 * 0.1) ≈ 1.34 x 10^-3
This value of x represents the equilibrium concentration of H+ ions. Finally, we can calculate the pH:
pH = -log(1.34 x 10^-3) ≈ 2.87
Therefore, the pH of a 0.1 M acetic acid solution is approximately 2.87.
These examples demonstrate the fundamental calculations involved in understanding the behavior of acids and bases.
Beyond the Basics: Practical Applications of Acids and Bases
Understanding acid-base chemistry goes beyond theoretical calculations. It has far-reaching implications in various fields:
- Medicine: pH plays a crucial role in maintaining the body’s delicate balance. For instance, the pH of blood is tightly regulated within a narrow range, and any fluctuation can lead to serious health conditions.
- Agriculture: Soil pH is critical for optimal plant growth. Different plants have specific pH preferences, and understanding soil acidity allows farmers to adjust soil conditions to support specific crops.
- Industrial Processes: Acids and bases are extensively used in manufacturing various products. For example, sulfuric acid is a key component in the production of fertilizers, detergents, and batteries.
- Environmental Concerns: Acid rain, caused by the release of sulfur dioxide and nitrogen oxides into the atmosphere, poses a significant environmental threat, impacting aquatic ecosystems and contributing to the corrosion of buildings and infrastructure.
Practice Makes Perfect: Mastering Acid-Base Calculations
Mastering acids and bases calculations requires consistent practice. You can access numerous online resources, textbooks, and practice worksheets to solidify your understanding.
Here are a few tips to improve your problem-solving skills:
- Start with basic concepts: Make sure you have a solid grasp of the core definitions, equations, and terminology before diving into complex calculations.
- Break down problems: Complex acid-base problems can often be broken down into smaller, manageable steps.
- Visualization: Draw diagrams or use visual aids to better understand the chemical processes involved.
- Seek guidance: If you encounter difficulties, consult your textbook, online resources, or seek help from a teacher or tutor.
Acids And Bases Calculations Practice Worksheet
Conclusion: A Journey of Chemical Exploration
Understanding acids and bases is an essential stepping stone for anyone delving into the fascinating world of chemistry. By mastering the key concepts, equations, and practice problems, you can unlock the secrets of chemical reactions and gain a deeper appreciation for the intricate interplay of acids and bases in our daily lives. Remember, practice is key to mastering any skill, and with the right tools and guidance, you can confidently navigate the realm of acid-base calculations. So, don’t be afraid to explore, to experiment, and to embrace the exciting challenges that chemistry has to offer.



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

