Have you ever wondered how seemingly disparate elements come together to form the intricate tapestry of the universe? The answer lies in the fascinating realm of chemical bonds, the invisible forces that govern the interactions between atoms. From the air we breathe to the water we drink, countless molecules exist thanks to these fundamental interactions. Understanding chemical bonds is not just a theoretical pursuit, it’s a key to unlocking the secrets of matter and its behavior, crucial in fields like medicine, materials science, and even everyday life. This guide serves as your comprehensive companion to mastering the intricacies of chemical bonding, equipping you with the knowledge to navigate answer keys, conquer worksheets, and ultimately, grasp the building blocks of the world around us.

Image: www.worksheeto.com
Imagine a world devoid of chemical bonds. Imagine no water to quench our thirst, no oxygen to sustain our lives, no vibrant colors to adorn our world. It’s a stark reality without the invisible forces that bind atoms together. These invisible forces, known as chemical bonds, are the very essence of molecular existence, driving the reactions that shape our universe. They are the architects of virtually everything we see, touch, and experience. This article dives deep into the world of chemical bonds, demystifying their various types and providing a complete understanding of the associated answer keys and worksheets that help solidify your understanding.
Delving into the Realm of Chemical Bonds
The concept of chemical bonding can be a bit daunting, but it’s best understood by visualizing atoms as tiny, intricate building blocks. Each atom comprises a nucleus, containing protons and neutrons, orbited by electrons that occupy distinct energy levels. These electrons, particularly those in the outermost shell, are the primary players in chemical bonding. The fundamental principle behind chemical bonding lies in the pursuit of stability. Atoms strive to achieve a stable configuration, resembling those of noble gases, with a complete outermost electron shell. This pursuit manifests in the formation of chemical bonds, as atoms share or transfer electrons, bringing them closer to attaining a noble gas configuration.
Types of Chemical Bonds: A Comprehensive Overview
Several types of chemical bonds govern the interactions between atoms, each with its unique characteristics and defining features. These include:
1. Ionic Bonds: The Transfer of Electrons
Ionic bonds are like a game of give and take, where one atom generously donates an electron to another, resulting in a charge imbalance. The atom that loses an electron becomes positively charged, forming a cation, while the atom that gains an electron becomes negatively charged, forming an anion. These oppositely charged ions are then attracted to each other through electrostatic forces, forming an ionic bond. These bonds are typically found in compounds composed of metals and nonmetals, like sodium chloride (NaCl), better known as table salt.
2. Covalent Bonds: The Sharing of Electrons
In contrast to ionic bonds, covalent bonds involve a more collaborative approach, where two atoms share one or more pairs of electrons. These shared electrons are attracted to the nuclei of both atoms, effectively holding them together. The attraction can be equal or unequal, depending on the electronegativity of the atoms involved. Covalent bonds are the heart of organic chemistry, forming the backbone of the countless molecules that make up our bodies and the world around us. Examples include water (H2O) and methane (CH4).
3. Metallic Bonds: A Sea of Electrons
Metallic bonds are unique in their ability to explain the characteristic properties of metals like their malleability, ductility, and excellent conductivity. In metallic bonds, the outermost electrons of metal atoms are delocalized, moving freely throughout the metallic lattice. This creates a “sea of electrons” that holds the positively charged metal ions together. These bonds also explain the unique bonding properties of alloys, where different metals are mixed to create new materials with desirable characteristics.
4. Hydrogen Bonds: The Special Case
Hydrogen bonds are a special kind of dipole-dipole interaction, where a hydrogen atom covalently linked to a highly electronegative atom, like oxygen or nitrogen, forms a weak bond with another electronegative atom. These bonds, while weak individually, are crucial in molecular interactions, influencing properties like water’s high boiling point and its ability to act as a solvent.
Answer Key Types of Chemical Bonds Worksheet: A Comprehensive Guide
Mastering chemical bonds is often facilitated by practice worksheets and answer keys. These invaluable tools provide students with a platform to test their knowledge, explore the nuances of bonding principles, and identify areas that require further attention.
1. Identifying Types of Bonds
Many worksheets focus on identifying the type of bond present in a given molecule. This requires students to examine the electronegativity difference between atoms, look for the presence of metals or nonmetals, and consider the sharing or transfer of electrons. Answer keys provide solutions by highlighting the key features of each bond type and explaining why a particular molecule exhibits a specific bond.
2. Predicting Chemical Properties
Another common practice involves predicting a molecule’s properties based on its bonding type. For instance, understanding the strength and polarity of a chemical bond allows for predictions regarding a molecule’s melting point, boiling point, solubility, and overall reactivity. Answer keys serve as a roadmap, guiding students through the interpretation of bond characteristics and their impact on chemical properties.
3. Drawing Lewis Structures
Lewis structures are visual representations of molecules, depicting the arrangement of atoms and the sharing of electrons. Many worksheets focus on drawing Lewis structures, emphasizing the role of valence electrons in bond formation. Answer keys provide the correct Lewis structures, allowing students to compare their work and identify any misconceptions or errors in their understanding of electron distribution and bond formation.
4. Delving into Molecular Geometry
Molecular geometry, or the three-dimensional arrangement of atoms in a molecule, plays a significant role in determining a molecule’s properties. Some worksheets explore the prediction of molecular geometry based on the type of bond and the presence of lone pairs of electrons on central atoms. Answer keys serve as a reference point, providing the correct molecular geometries and illustrating the impact of electron repulsion on the spatial arrangement of atoms.
5. Applying Bonding Principles to Real-World Applications
These worksheets challenge students to apply their understanding of chemical bonds to real-world phenomena. For example, they may be asked to explain the properties of various materials, such as why diamonds are exceptionally hard or why metals are excellent conductors. Answer keys provide explanations that connect bonding principles to practical applications, demonstrating the relevance of chemical bonding in shaping our world.
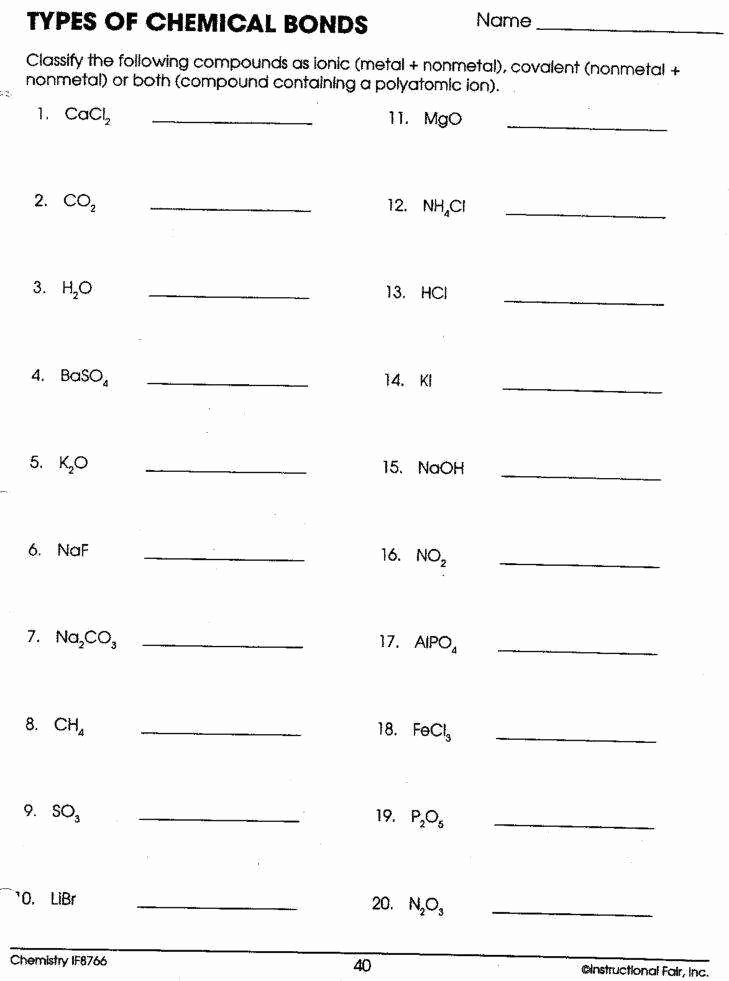
Image: chessmuseum.org
Expertise and Actionable Tips for Success
Mastering chemical bonds requires a blend of understanding foundational concepts, applying them to diverse situations, and embracing a curious spirit to explore the universe of molecules.
1. Engage in Active Learning: The key to success lies in actively engaging with the material. Instead of passively reading about chemical bonds, try to visualize the interactions between atoms and draw Lewis structures.
2. Embrace Visualization: Whenever possible, visualize the concept of bonding. Imagine atoms as tiny spheres with electrons orbiting them, and envision the bonds as strings linking them together.
3. Seek Peer Collaboration: Working with classmates can be immensely helpful. Discuss challenging concepts, compare answers, and learn from each other’s perspectives.
4. Utilize Online Resources: There are numerous online resources, including animations, videos, and interactive simulations, that can aid in conceptualizing complex topics like chemical bonds. Explore them to enhance your understanding.
5. Seek Guidance from Experts: If you encounter obstacles, don’t hesitate to seek guidance from your instructor or a tutor. They can provide personalized explanations and address your specific questions.
Answer Key Types Of Chemical Bonds Worksheet
Conclusion: Unlocking the Universe of Molecules
Understanding chemical bonds is fundamental to unraveling the mysteries of the world around us. It helps us comprehend the structures of molecules, predict their behavior, and understand the forces that govern chemical reactions. By delving into answer keys and worksheets, we embark on a journey of exploration and discovery, solidifying our knowledge and pushing the frontiers of what we know. So, embrace the challenge, explore the intricacies of chemical bonds, and unlock the universe of molecules, one bond at a time. With practice, curiosity, and a touch of scientific wonder, you too can master the art of chemical bonding!



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

