Imagine a world where you could precisely measure the strength of an acid or base with just a drop of another solution. It might sound like magic, but this is the power of neutralization titration – a fundamental chemistry experiment that unlocks the secrets of acid-base reactions. Experiment 22, often tackled in introductory chemistry courses, delves into the intricacies of this technique, allowing students to gain hands-on experience in understanding chemical reactions at a molecular level.

Image: www.chegg.com
This article serves as your comprehensive guide to navigating the world of Experiment 22: Neutralization Titration 1. We’ll decipher the underlying principles, explore the experimental procedure, and unravel the meaning behind those all-important answers. Whether you’re a student preparing for your next lab session or a curious mind seeking to understand the chemistry of everyday life, this guide will equip you with the knowledge and insights to master this rewarding experiment.
A Journey into the Heart of Acid-Base Reactions
At the core of Experiment 22 lies the fascinating realm of acid-base reactions. These reactions involve the transfer of hydrogen ions (H+) between molecules, ultimately influencing the pH of a solution. Acids, with their characteristic sour taste and ability to donate H+ ions, clash with bases, known for their bitterness and penchant for accepting H+ ions. Think of it like a chemical tug-of-war, where acids try to pull away H+ ions, and bases attempt to grab hold of them.
When an acid and a base come face-to-face, they embark on a dance of neutralization, resulting in the formation of salt and water. This dance is governed by the concept of stoichiometry, the language of chemical proportions. Neutralization titration serves as a powerful tool to measure the precise amount of acid or base needed to achieve this perfect balance.
The Chemistry Behind the Lab: Unraveling the Concepts
In the world of Experiment 22: Neutralization Titration 1, you will encounter several key players that shape the experiment’s outcome:
Titrant: This is the solution of known concentration, carefully added drop by drop to the analyte (the solution of unknown concentration). Imagine it as the detective, slowly searching for the right amount needed to completely react with the analyte.
Analyte: The solution of unknown concentration, waiting to be neutralized by the titrant. This is the mystery ingredient, whose concentration we are eager to unveil.
Equivalence Point: The moment of truth! This is the point where the titrant and analyte have reacted in perfect stoichiometric proportions, resulting in complete neutralization. This equilibrium point is signaled by a color change in the indicator, revealing the exact amount of acid or base needed for complete neutralization.
Indicator: This chemical chameleon, added to the analyte, changes color in response to the pH change as the titration progresses. It acts as a visual guide, signaling when the equivalence point has been reached.
Step-by-Step: Executing the Titration
Experiment 22: Neutralization Titration 1 unfolds in a series of meticulously planned steps:
-
Preparation: Accurately measure out the analyte and add a few drops of indicator. Fill the burette with the titrant, ensuring its initial volume is clearly marked.
-
Titration: Carefully add the titrant to the analyte, drop by drop, while constantly swirling the solution. Keep a watchful eye on the color change of the indicator.
-
Equivalence Point Determination: Stop titrating the moment the indicator undergoes a permanent color change, indicating the equivalence point has been reached. This color change is a visual marker, telling you the titrant and analyte have reacted completely.
-
Data Analysis: Record the initial and final burette readings, the volume of titrant used, and the concentration of the titrant. Using these data, you can calculate the concentration of the unknown analyte.
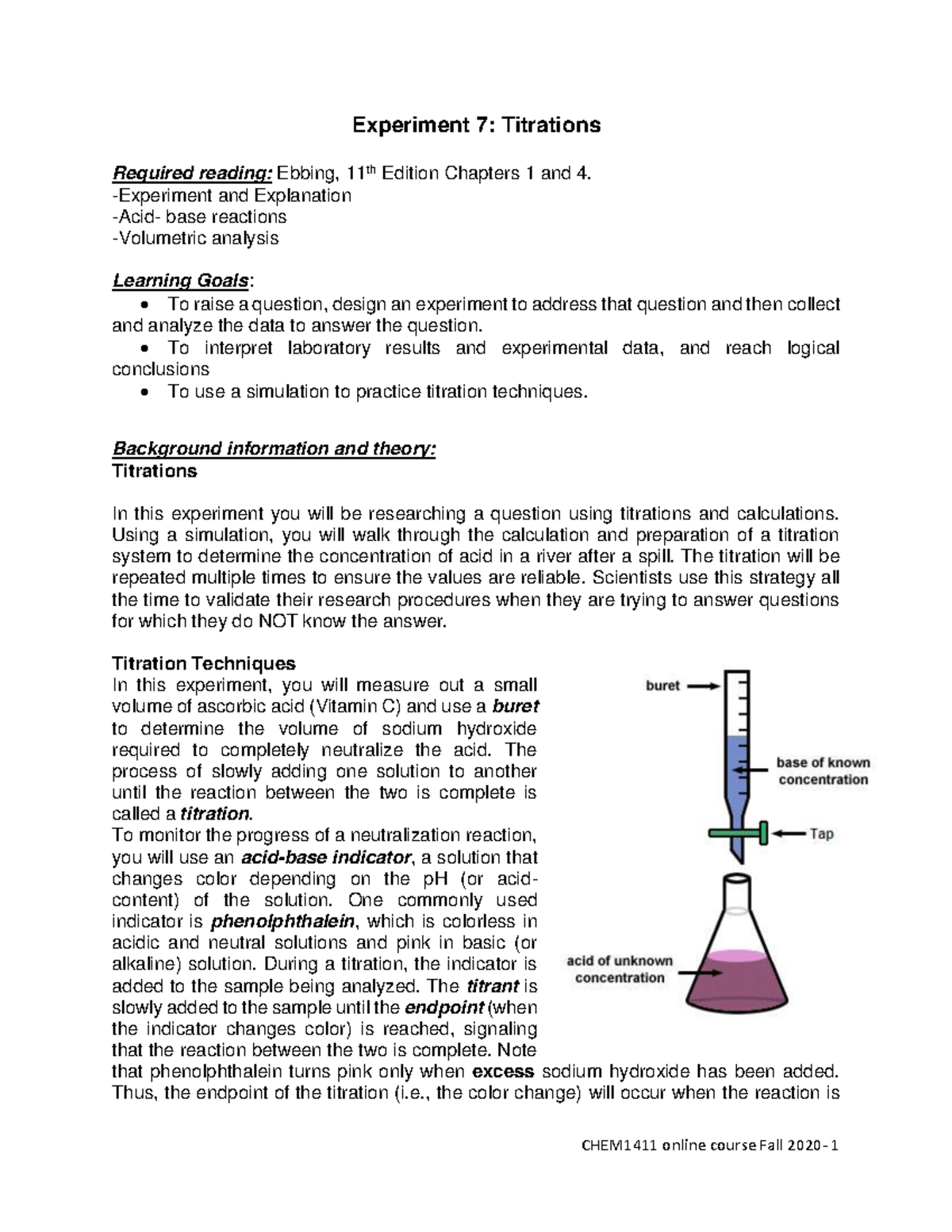
Image: www.studocu.com
Interpreting the Results: Demystifying the Answers
The beauty of Experiment 22 lies in the ability to unlock the concentration of the unknown analyte. By understanding the stoichiometry of the reaction and the volume of titrant used, you can calculate the concentration of the unknown solution. The answers you obtain are more than just numbers; they represent the culmination of careful titration techniques, precise measurements, and a deep understanding of chemical reactions.
Common Challenges and Troubleshooting Tips
As with any experiment, Experiment 22: Neutralization Titration 1 can present its share of challenges. Don’t be discouraged! There are several common issues and troubleshooting tips to help you navigate these potential hurdles.
Challenge: Inaccurate Measurement:
Solution: Precision is key in titration. Ensure you measure out the analyte and titrant accurately, using calibrated measuring equipment.
Challenge: Incomplete Neutralization:
Solution: Titrate slowly, especially as you approach the equivalence point. Swirl the solution to ensure proper mixing and prevent premature color changes.
Challenge: Wrong Indicator Selection:
Solution: Choose an indicator with a color change in the pH range that corresponds to the equivalence point of your particular acid-base reaction.
Challenge: Incorrect Data Recording:
Solution: Record your data meticulously, including the initial and final burette readings, volume of titrant used, and the concentration of the titrant. Avoid errors during data entry.
Expert Insights to Elevate Your Titration
To further enhance your understanding and success in Experiment 22, let’s turn to expert insights from the field of chemistry:
Dr. Emily Carter, a renowned chemist, emphasizes the importance of proper technique: “Accuracy is crucial for successful titrations. Practice precision in your pipetting and burette readings, and always double-check your calculations.”
Professor William Anderson, a veteran chemistry instructor, stresses the importance of understanding the theoretical concepts: “Don’t just go through the motions. Take the time to understand the principles behind neutralization titration. This will make the experiment much more meaningful and help you interpret the results with greater confidence.”
Putting it All Together: Using Your Knowledge to Make a Difference
Experiment 22: Neutralization Titration 1 is more than a lab exercise; it’s a gateway to a world of scientific understanding. The knowledge you gain from this experiment has broad applications, extending beyond the laboratory and into everyday life:
-
Understanding pH in your Kitchen: Neutralization titration becomes a powerful tool for analyzing the acidity and alkalinity of everyday substances, such as vinegar, baking soda, and fruits.
-
Monitoring Water Quality: Using titration techniques, we can measure the acidity or alkalinity of water sources, ensuring the safety of our drinking water.
-
Developing Pharmaceutical Solutions: Neutralization titration forms the foundation for pharmaceutical drug development, ensuring precise dosages and stability of medications.
Experiment 22 Neutralization Titration 1 Answers
The End of the Journey, the Beginning of Exploration
Experiment 22: Neutralization Titration 1 offers more than just an introduction to titration; it’s a springboard. The skills and knowledge you gain from this experiment can be applied to more advanced titration techniques, opening doors to a deeper understanding of chemical reactions and their applications.
As you delve into the world of chemistry, remember that every experiment, every equation, every titration, represents a journey into a deeper understanding of the universe around us. Embrace the challenge, celebrate the discoveries, and keep asking questions. The world of science is brimming with wonders waiting to be unveiled!



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

