Ever wondered how rust forms on a metal fence, or how baking soda makes your cookies rise? These everyday phenomena are fascinating examples of chemical reactions happening right before our eyes. Chemical reactions are the heart of chemistry, the transformations that rearrange atoms and molecules, creating new substances and shaping our world. Understanding these reactions is crucial, and a great way to grasp the fundamentals is through interactive exercises like worksheets. These worksheets, combined with their answer keys, serve as potent tools for learning and solidifying knowledge about the different types of chemical reactions.

Image: worksheetpic101.s3.amazonaws.com
Chemical reactions are classified into different types based on the changes occurring in the chemical bonds of the reactants. This classification helps us predict the products, understand the energy changes involved, and even control the reaction conditions. Let’s embark on a journey through the common types of chemical reactions, exploring their key characteristics, using worksheet examples as our guide.
1. Synthesis Reactions: Building Blocks of Chemistry
Imagine Lego bricks, small individual pieces that can be combined to build complex structures. Synthesis reactions, also known as combination reactions, are similar. They start with two or more simple reactants and combine them to form a single, more complex product. A famous example is the formation of water, where two hydrogen atoms and one oxygen atom come together to create the life-sustaining molecule H2O.
Here’s a typical worksheet problem illustrating a synthesis reaction:
Example 1:
Reactants: Sodium (Na) + Chlorine (Cl2)
Product: Sodium chloride (NaCl)
Answer Key: The reaction is represented as: 2Na + Cl2 –> 2NaCl
The worksheet would likely ask you to write the balanced chemical equation, which ensures the same number of atoms of each element are present on both sides of the reaction.
2. Decomposition Reactions: Breaking Down into Simpler Forms
Decomposition reactions are the reverse of synthesis, where a complex compound breaks down into simpler substances. It’s like taking a complex puzzle apart, separating it into its individual pieces. A familiar example is the breakdown of limestone (CaCO3) into calcium oxide (CaO) and carbon dioxide (CO2) under heat.
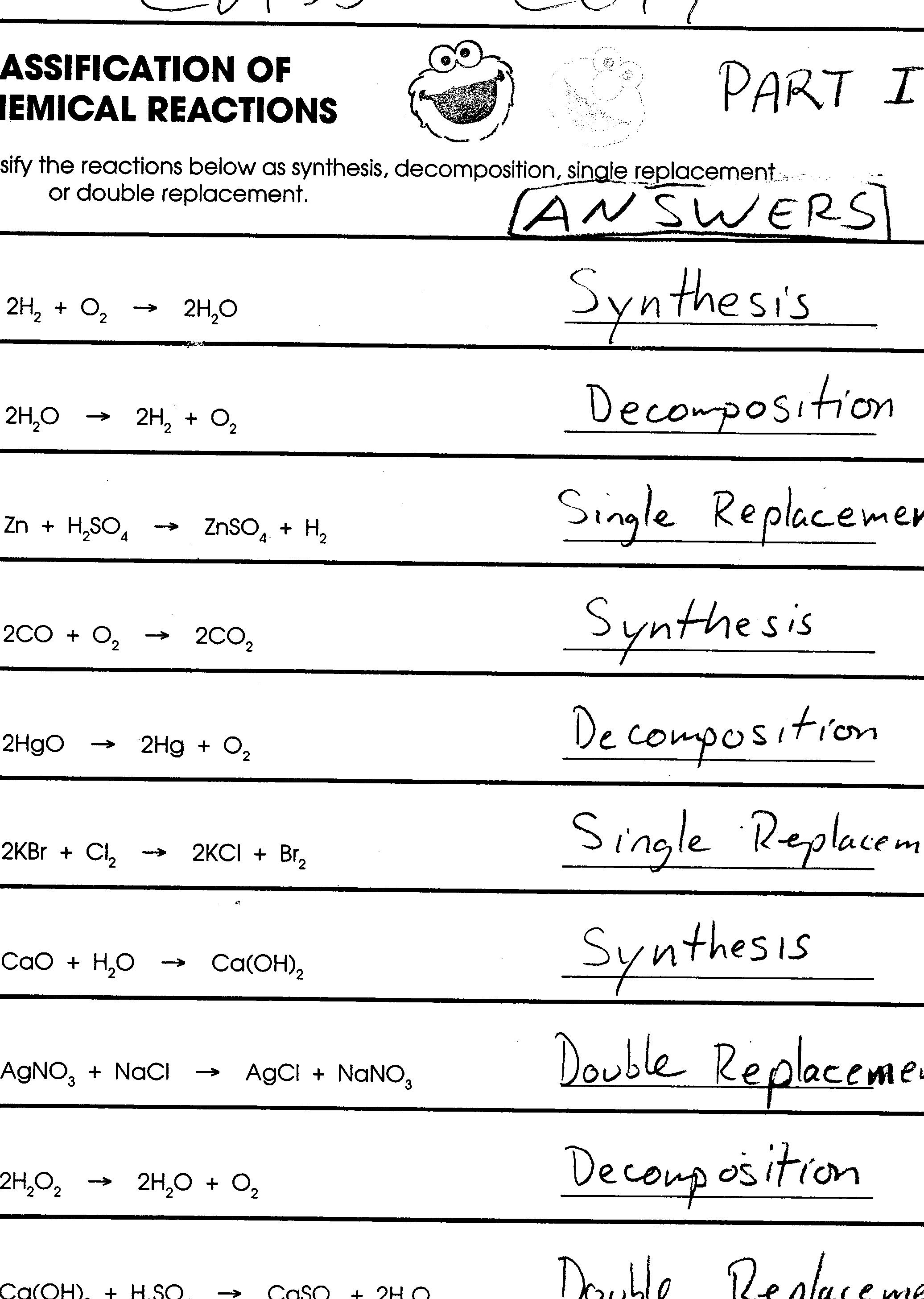
Image: bitrix.informator.ua
Example 2:
Reactant: Hydrogen peroxide (H2O2)
Products: Water (H2O) + Oxygen (O2)
Answer Key: The balanced equation is: 2H2O2 –> 2H2O + O2
This reaction is commonly used in household applications, where it decomposes to release oxygen, which can disinfect wounds and whiten teeth.
3. Single Displacement Reactions: One Element Takes the Place of Another
In the bustling world of chemistry, elements are always vying for positions. Single displacement reactions involve one element displacing another from a compound. Imagine a bolder element pushing its way into a compound, replacing a weaker element. Zinc reacting with hydrochloric acid is a classic example. The zinc displaces hydrogen from the acid, producing hydrogen gas and zinc chloride:
Zn + 2HCl –> ZnCl2 + H2
Example 3:
Reactants: Iron (Fe) + Copper(II) sulfate (CuSO4)
Products: Iron(II) sulfate (FeSO4) + Copper (Cu)
Answer Key: The balanced equation is:
Fe + CuSO4 –> FeSO4 + Cu
This reaction highlights the reactivity series of metals. More reactive metals, like zinc, can displace less reactive metals, like copper, from their compounds.
4. Double Displacement Reactions: Partners Swap Places
Double displacement reactions involve a swap of partners, with two compounds reacting to exchange ions, creating two new compounds. It’s like a dance where partners switch places, forming new pairings. A familiar example is the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) to produce silver chloride (AgCl) and sodium nitrate (NaNO3):
AgNO3 + NaCl –> AgCl + NaNO3
Example 4:
Reactants: Potassium iodide (KI) + Lead(II) nitrate (Pb(NO3)2)
Products: Potassium nitrate (KNO3) + Lead(II) iodide (PbI2)
Answer Key: The balanced equation is:
2KI + Pb(NO3)2 –> 2KNO3 + PbI2
This reaction is a common method for creating insoluble precipitates (solids that form from a solution). Lead(II) iodide forms a bright yellow precipitate, providing a visual indication that a reaction has occurred.
5. Combustion Reactions: A Symphony of Flame and Energy
Combustion reactions are the hallmark of energy production. They involve the rapid reaction between a substance with an oxidant, typically oxygen, releasing a significant amount of energy in the form of heat and light. This is what fuels our cars, power plants, and even the flames in our homes. The burning of propane (C3H8) in a grill is a prime example:
C3H8 + 5O2 –> 3CO2 + 4H2O
Example 5:
Reactants: Methane (CH4) + Oxygen (O2)
Products: Carbon dioxide (CO2) + Water (H2O)
Answer Key: The balanced equation is:
CH4 + 2O2 –> CO2 + 2H2O
These reactions are vital for our energy needs and have significant environmental implications. Many efforts are focused on developing cleaner combustion technologies to minimize harmful emissions.
Types Of Chemical Reaction Worksheet Answer Key
Unveiling the Secrets of Chemical Reactions
Understanding the different types of chemical reactions is like having a key to unlocking the complex world of chemistry. Worksheets and their answer keys provide a structured framework for learning, allowing you to practice identifying reactions, writing balanced equations, and applying fundamental chemical principles. Remember, chemical reactions are the foundation of life, governing everything from the synthesis of food in plants to the breakdown of materials in our bodies. By embracing these concepts, you can begin to appreciate the intricate dance of atoms and molecules that shape our world.
Don’t hesitate to explore further! There are countless resources available to deepen your understanding of chemical reactions, from online tutorials to laboratory experiments. The journey into the world of chemistry is full of fascinating discoveries waiting to be made.



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

