Imagine you’re a chemist in a bustling laboratory, meticulously measuring the pressure, volume, and temperature of a gas. Suddenly, a question pops into your head: How are all these factors connected? How can you predict the behavior of this gas, like a skilled conductor leading an orchestra of molecules? The answer lies in the heart of chemistry: the Ideal Gas Law. It’s a powerful tool that allows us to understand and predict the behavior of ideal gases, those that perfectly obey the law. But navigating the intricacies of the Ideal Gas Law can feel like deciphering a complex map. That’s where packet worksheets come in. These helpful guides, filled with questions and practice problems, can act as your trusty companion on this fascinating journey through the world of gases.

Image: lessonfullaachen.z1.web.core.windows.net
This article is your guide to conquering the Ideal Gas Law and its associated packet worksheets. We’ll dive deep into the fundamentals of this law, exploring its history, its crucial components, and its real-world applications. We’ll unravel the mysteries of those packet worksheets, demystifying common problems and equipping you with the tools to confidently tackle any challenge thrown your way. So, put on your lab coat, grab your trusty calculator, and let’s embark on this enriching exploration together!
A Journey Back in Time: The Roots of the Ideal Gas Law
The Ideal Gas Law didn’t just appear out of thin air. Its story is woven into the fabric of scientific discovery, shaped by the contributions of brilliant minds throughout history. In the 17th century, the ingenious Robert Boyle laid the groundwork by observing the relationship between pressure and volume in a gas. This relationship, now known as Boyle’s Law, states that at a constant temperature, the pressure and volume of a gas are inversely proportional. In simpler terms, if you squeeze a gas into a smaller volume, its pressure will increase.
The next key piece of the puzzle was added by the equally brilliant Jacques Charles in the late 18th century. His experiments with gases at different temperatures revealed that at a constant pressure, the volume of gas is directly proportional to its absolute temperature. This means if you heat up a gas, its volume will expand. These groundbreaking discoveries were later formalized into Charles’s Law.
Finally, in the early 19th century, Avogadro’s Law contributed another crucial piece to the puzzle. This law states that at constant pressure and temperature, equal volumes of gases contain the same number of molecules.
These three laws, each meticulously derived through observation and experimentation, paved the way for the culmination of our understanding: the Ideal Gas Law. It beautifully combines these individual laws into one elegant equation: PV = nRT, where:
- P represents the pressure of the gas
- V represents the volume of the gas
- n represents the number of moles of gas
- R is the ideal gas constant (a constant value used in the Ideal Gas Law)
- T represents the absolute temperature of the gas
Decoding the Ideal Gas Law: A Closer Look
The Ideal Gas Law, as its name suggests, describes the behavior of ideal gases. These theoretical gases are composed of point particles that don’t interact with each other except for perfectly elastic collisions. While we are working with ideal gases theoretically, this law provides a remarkably accurate understanding of the behavior of real gases under normal conditions.
The equation itself is surprisingly versatile. It allows us to solve for any of the variables, given the other three. For instance, if we know the pressure, volume, and temperature of a gas, we can calculate the number of moles present using the Ideal Gas Law.
Mastering the Ideal Gas Law Worksheet: Your Guide to Success
Packet worksheets are designed to guide you through the Ideal Gas Law, providing practice problems and step-by-step explanations to solidify your understanding. Often, these worksheets contain a variety of problem types, each designed to focus on a specific aspect of the Ideal Gas Law.
Here are some common types of problems you might encounter:
- Predicting the unknown variable: These problems provide you with three out of the four variables in the Ideal Gas Law equation (P, V, n, T) and ask you to solve for the unknown variable.
- Converting Units: The Ideal Gas Law requires specific units for its variables (e.g., pressure in atmospheres, volume in liters, temperature in Kelvin). These problems will often test your ability to convert between different units.
- Stoichiometry Problems: These problems connect the Ideal Gas Law to other chemical concepts, such as stoichiometry, asking you to calculate the amount of gas produced or consumed in a chemical reaction.
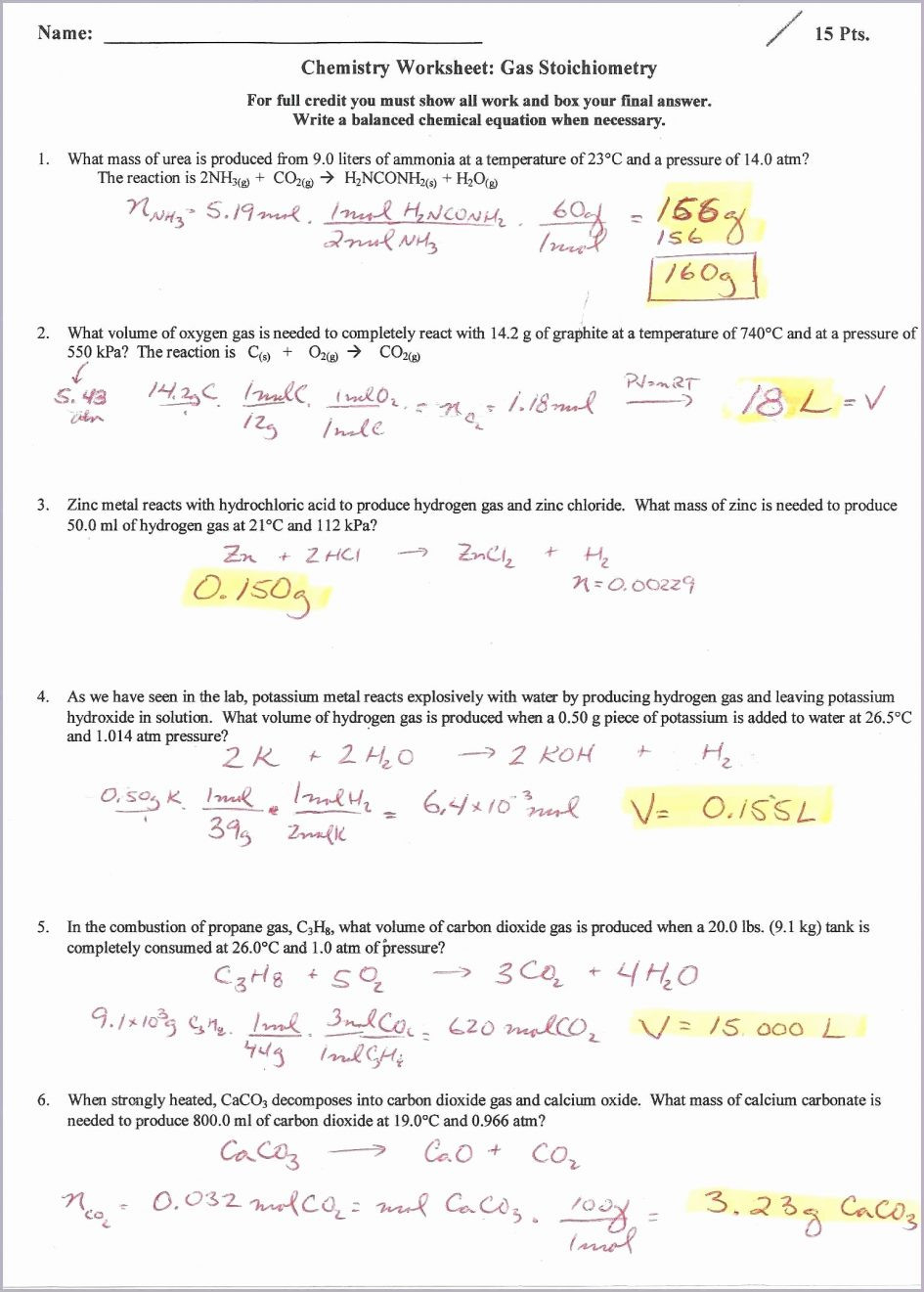
Image: db-excel.com
Unlocking the Secrets: Strategies for Tackling Ideal Gas Law Worksheets
Tackling Ideal Gas Law worksheets can feel daunting, but remember, practice makes perfect! Here are some key strategies to help you conquer these problems:
- Read the problem carefully: Take the time to understand what the problem is asking, identifying the known variables and the unknown one.
- Choose a suitable equation: Select the appropriate formula based on the question. For most problems, the Ideal Gas Law (PV = nRT) will be your go-to equation.
- Watch your units: Always make sure that your variables are expressed in the correct units. If necessary, convert them before plugging them into the equation.
- Show your work: Write down each step of your calculations clearly and meticulously. This will help you track your work and identify any mistakes.
- Practice, practice, practice: There’s no better way to master the Ideal Gas Law than by working through numerous practice problems. Utilize the examples in your textbook, online resources, and, of course, those packet worksheets!
Beyond the Worksheets: Real-World Applications of the Ideal Gas Law
The Ideal Gas Law isn’t just a theoretical exercise; it shapes various aspects of our daily lives.
- Weather Forecasting: Meteorologists utilize the Ideal Gas Law to understand atmospheric pressure changes and predict weather patterns.
- Automotive Industry: The Ideal Gas Law is vital for designing and optimizing internal combustion engines, helping engineers understand how gases behave within the engine cylinders.
- Medical Applications: The Ideal Gas Law finds its use in medical devices like ventilators, which rely on the principles of gas pressure and volume for delivering oxygen to patients.
- Industrial Chemistry: The Ideal Gas Law is essential in chemical engineering, enabling calculations involving gas volumes, pressures, and reaction rates.
Expert Insights and Actionable Tips
Dr. Maria Sanchez, a renowned chemistry professor, emphasizes the importance of a strong foundation in the Ideal Gas Law: “Mastering the Ideal Gas Law opens doors to a deeper understanding of chemistry. It’s a versatile tool that can be applied to a wide range of problems, from simple laboratory experiments to complex industrial processes.”
Here’s a tip from Dr. Sanchez for tackling Ideal Gas Law problems: “Remember, the Ideal Gas Law is a fundamental relationship between pressure, volume, temperature, and the number of moles of gas. Understanding the concept of proportionality between these variables is key to solving problems effectively.”
Ideal Gas Law Packet Worksheet Answers
Conclusion
The Ideal Gas Law, often seemingly intimidating, can be a fascinating and powerful tool once you understand its intricacies. Packet worksheets, though initially daunting, serve as your roadmap through this journey of discovery. As you delve into these worksheets, remember that each problem is an opportunity to grow your knowledge of this fundamental law. By understanding its historical roots, mastering its applications, and challenging yourself with practice problems, you’ll unlock the secrets of the Ideal Gas Law and become a confident gas maestro.
Now, go forth, dear reader, and equip yourself with the knowledge you’ve gained. Let your journey through the world of gases be filled with confidence and discovery!



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

