Have you ever wondered how tiny atoms, the building blocks of everything around us, can create such a diverse and fascinating world? The answer lies in their intricate internal structure, particularly in the arrangement of their electrons. Understanding this arrangement, known as electron configuration, is crucial for unraveling the secrets of chemical bonding, predicting reactivity, and explaining the properties of elements.

Image: ataglance.randstad.com
This article delves into the realm of electron configuration, focusing on POGIL (Process Oriented Guided Inquiry Learning) worksheets designed to empower you to master this fundamental concept in chemistry. Prepare to embark on an interactive journey that will shed light on the fascinating interplay between electrons, energy levels, and the periodic table.
Exploring the POGIL Approach
Before diving into electron configuration itself, let’s understand the power of POGIL worksheets. POGIL, a student-centered learning strategy, encourages active participation and collaborative learning. Instead of passively receiving information, students are challenged to investigate concepts, solve problems, and arrive at conclusions through guided discussion and critical thinking. The POGIL methodology helps learners internalize knowledge while developing essential problem-solving skills.
Delving into Electron Configuration
Every atom consists of a dense, positively charged nucleus surrounded by negatively charged electrons. These electrons occupy specific energy levels, also called electron shells, which exist at different distances from the nucleus. Each energy level can accommodate a maximum number of electrons, determined by a set of rules and principles.
The fundamental groundwork for understanding electron configuration lies in the following key concepts:
1. The Aufbau Principle: Filling the Shells
The Aufbau principle is like a map for filling electrons into energy levels and sublevels. It states that electrons are added one at a time to the lowest available energy level, gradually building up the electron configuration of an atom. Imagine it like assembling a jigsaw puzzle, starting with the smallest pieces and working your way to larger ones.
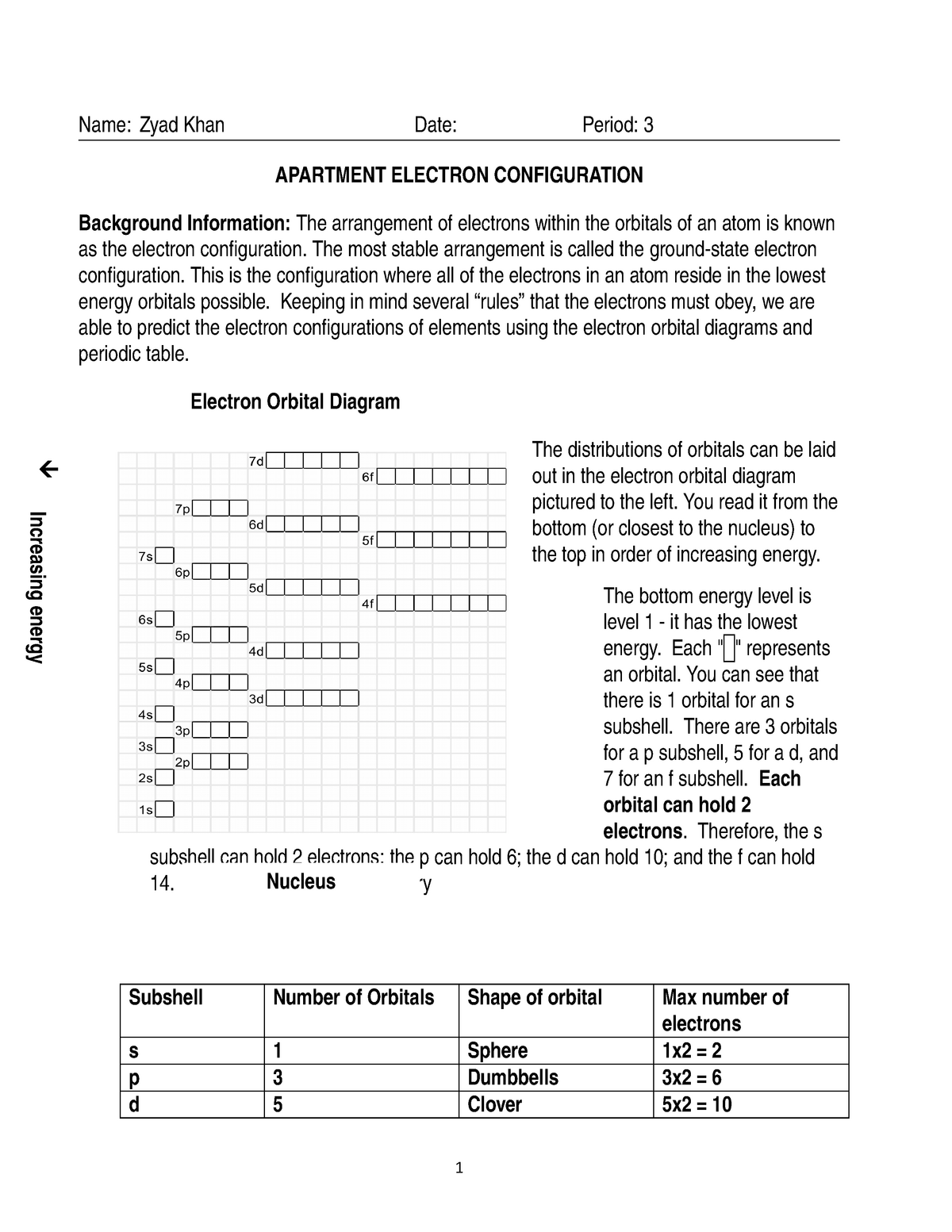
Image: domque.blogspot.com
2. The Hund’s Rule: Democratizing Orbitals
Within each energy level, there are sublevels called orbitals. Each orbital can accommodate a maximum of two electrons. Hund’s rule dictates that electrons prefer to occupy different orbitals within a sublevel before pairing up within the same orbital. Think of each orbital as a separate seat on a bus: electrons will individually occupy different seats before doubling up!
3. The Pauli Exclusion Principle: No Sharing!
The Pauli exclusion principle states that no two electrons can have the same set of four quantum numbers. Quantum numbers define the specific properties of an electron, including its energy level, orbital, and spin. This principle prevents electron overcrowding and ensures that each electron in an atom has a unique identity.
Unlocking the POGIL’s Potential: A Case Study
Imagine a POGIL worksheet that explores the electron configurations of the first 20 elements. The worksheet might start with a table listing the elements and their atomic numbers. Students would then be tasked with collaborating to determine the electron configurations of each element, following a step-by-step process guided by the principles discussed above. For instance, they would need to:
- Determine the number of energy levels needed to accommodate the element’s electrons.
- Identify the maximum number of electrons allowed in each energy level.
- Use Hund’s rule and the Aufbau principle to fill the energy levels and orbitals systematically.
- Apply the Pauli exclusion principle to ensure each electron has a unique set of quantum numbers.
Through collaborative discussions and problem-solving, students gain a deeper understanding of electron configuration and its relationship to the periodic table. They learn to recognize patterns in electron configurations and connect these patterns to the elements’ properties.
Beyond the Worksheet: Applications in Chemistry
Understanding electron configuration is not just an academic exercise; it has profound implications in various chemical concepts and applications. Here are some examples:
1. Chemical Bonding: Explaining the Glue of Molecules
Electron configuration plays a pivotal role in explaining how atoms form chemical bonds to create molecules. When atoms interact, they tend to gain, lose, or share electrons to achieve a stable electron configuration, which typically involves a full outer shell of electrons. Understanding electron configuration helps predict how atoms will bond and the type of bond formed (ionic, covalent, or metallic).
2. Predicting Reactivity: Unveiling the Potential
The reactivity of an element is closely related to its electron configuration. Elements with unfilled outer shells are more likely to react with other elements to achieve a stable configuration. This is why alkali metals readily lose an electron to become positively charged ions, while halogens readily gain an electron to become negatively charged ions.
3. Explaining Properties: Unraveling the Puzzle of Diversity
Electron configuration explains the wide range of properties exhibited by elements. For instance, metals are good conductors of electricity and heat due to the presence of loosely held electrons in their outer shells. Nonmetals, on the other hand, are generally poor conductors due to tightly bound electrons in their outer shells. Understanding electron configuration helps us predict and explain these diverse properties.
The POGIL Advantage: A Personalized Learning Journey
The beauty of POGIL worksheets lies in their adaptability and personalized approach. They provide a structured framework for learning while allowing for differentiated instruction and individual pacing. Students can work collaboratively, supporting each other in navigating the complexities of electron configuration at a pace that suits their individual learning styles.
Electron Configuration Worksheet Pogil Answer Key
Conclusion: Embracing the Power of Electron Configuration
Mastering electron configuration is a stepping stone to a deeper understanding of chemistry. Through interactive POGIL worksheets, you can unravel the mysteries of atomic structure, gain insights into chemical behavior, and predict properties of elements. So, embrace the opportunity to explore the world of electron configuration armed with the knowledge and tools gained through these engaging and effective worksheets. You’ll be amazed at how this fundamental concept unlocks a world of chemical marvels!



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

