Remember those colorful diagrams in your chemistry textbook, with those tiny circles floating around a larger one? Those are representations of atoms, the fundamental building blocks of matter. But what exactly are those circles and what makes them so special? They’re protons, neutrons, and electrons, the particles that make up every atom in the universe. Grasping these concepts is key to understanding how everything around us works, and for many, a practice worksheet on protons, neutrons, and electrons can be a great way to solidify this knowledge.

Image: www.unmisravle.com
I vividly recall my first encounter with these atomic particles in high school chemistry. Our teacher had us build simple models of atoms using different colored balls to represent protons, neutrons, and electrons. While the exercise seemed simple enough, it sparked a fascination in me that led me down the rabbit hole of atomic physics. This is the kind of “aha” moment that these practice worksheets strive to ignite in students, making the abstract world of atoms more tangible and approachable.
The Tiny Building Blocks of Everything: Protons, Neutrons, and Electrons
Protons, neutrons, and electrons are the fundamental particles that make up every atom. These tiny particles, located within the nucleus of an atom, possess unique characteristics that define the identity and behavior of an element. While they may seem like abstract concepts at first glance, understanding these subatomic particles is actually quite simple.
To put things into perspective, visualize an atom as a miniature solar system with a central sun (the nucleus) and orbiting planets (electrons). The nucleus is composed of protons and neutrons, which are significantly heavier than electrons. Protons carry a positive electric charge, neutrons are neutral, and electrons possess a negative charge. These charges are what make atoms interact with each other and form molecules, the basis of all matter.
The Roles of Protons, Neutrons, and Electrons in Atomic Structure
Protons: The Identity Defining Particles
Protons are the heavyweights in the atomic world. They reside in the atom’s core, known as the nucleus, and hold a positive charge. The number of protons an atom possesses is called its atomic number and is the defining characteristic of an element. For instance, all carbon atoms have 6 protons, while all oxygen atoms have 8 protons.
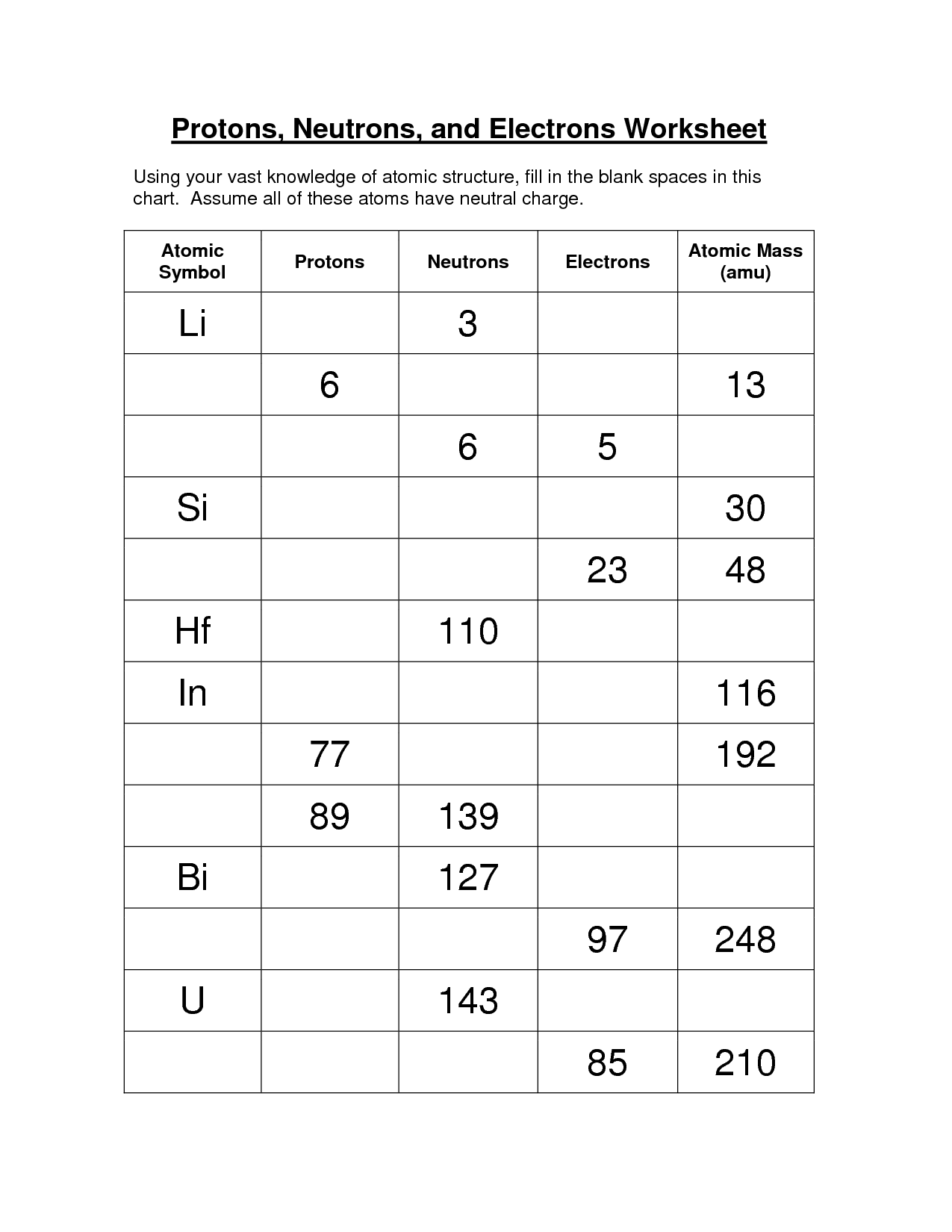
Image: byveera.blogspot.com
Neutrons: The Atomic Stabilizers
Neutrons, as their name suggests, are neutral particles found in the nucleus alongside protons. They don’t have a charge, but they play a crucial role in stabilizing the atom. The number of neutrons can vary within an element, resulting in different isotopes, which are atoms with the same atomic number but varying atomic masses.
Electrons: The Orbiting Minions
Electrons are the lightest of the subatomic trio and orbit around the nucleus in distinct energy levels called shells. They carry a negative charge and are responsible for chemical bonding, the process by which atoms combine to form molecules. The arrangement of electrons in an atom determines its chemical properties and reactivity.
Unlocking the Secrets of Atomic Structure with Practice Worksheets
Practice worksheets are essential tools for students to solidify their understanding of the intricate world of atomic structure. These worksheets typically present various exercises that involve identifying the number of protons, neutrons, and electrons in atoms, drawing simple atomic models, or solving for atomic mass and atomic number.
Through these exercises, students learn to apply the fundamental concepts they’ve learned about protons, neutrons, and electrons to real-world examples. This hands-on approach not only deepens their knowledge but also makes the subject matter more engaging and less daunting.
Tips for Mastering Protons, Neutrons, and Electrons
To navigate the world of protons, neutrons, and electrons, consider these tips:
- Visualize: Use mental images or physical models to represent atoms, and clearly distinct the locations of protons, neutrons, and electrons. This will aid in understanding their roles and relationships within the atom.
- Practice Regularly: Consistent practice is key to mastering any concept, especially in science. Regularly work through practice problems that involve calculating atomic numbers and masses, drawing atomic models, and identifying the different types of subatomic particles. The more you practice, the more comfortable you’ll become with these concepts.
- Utilize Online Resources: Numerous online websites offer interactive quizzes, games, simulations, and explanations that help make learning about protons, neutrons, and electrons more engaging and interactive. These resources can provide valuable support and supplement your textbook and practice worksheets.
- Don’t Hesitate to Ask for Help: If you encounter any difficulties, don’t hesitate to seek assistance from your teacher, classmates, or online resources. Everyone has their own way of learning, and there’s no shame in asking for help when you need it.
FAQs about Protons, Neutrons, and Electrons
Q: What’s the difference between an atom and a molecule?
A: An atom is the single, basic unit of an element, while a molecule is formed when two or more atoms bond together.
Q: Can the number of protons in an atom change?
A: No, the number of protons in an atom defines its element. Changing the number of protons would create a completely different element.
Q: Can the number of neutrons in an atom change?
A: Yes, varying the number of neutrons within an element creates different isotopes of that element.
Q: How do electrons determine chemical bonding?
A: Atoms tend to form bonds by sharing or transferring electrons, trying to achieve a stable outer shell of electrons. This process dictates how atoms interact and form molecules.
Protons Neutrons Electrons Practice Worksheet Answers
Conclusion: Embark on Your Journey into the Atomic World
Understanding the roles of protons, neutrons, and electrons is foundational to comprehending the nature of matter. Through practice worksheets and consistent learning, you can master the intricate workings of atomic structure. Remember, the journey to understanding the world around us starts with appreciating the tiny building blocks that make it all possible. So, are you ready to delve deeper into the fascinating world of atoms?



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

