The periodic table is a familiar sight in classrooms and laboratories around the world. It’s a powerful tool that helps us understand the properties of elements and how they interact with each other. But have you ever noticed that the atomic masses listed on the table aren’t always whole numbers? That’s because atomic mass is actually a weighted average of the masses of all the isotopes of an element. Isotopes are atoms of the same element that have different numbers of neutrons. This means that the atomic mass of an element can vary slightly depending on the relative abundance of its isotopes. But for many practical applications, it’s useful to round the atomic mass to the nearest whole number.
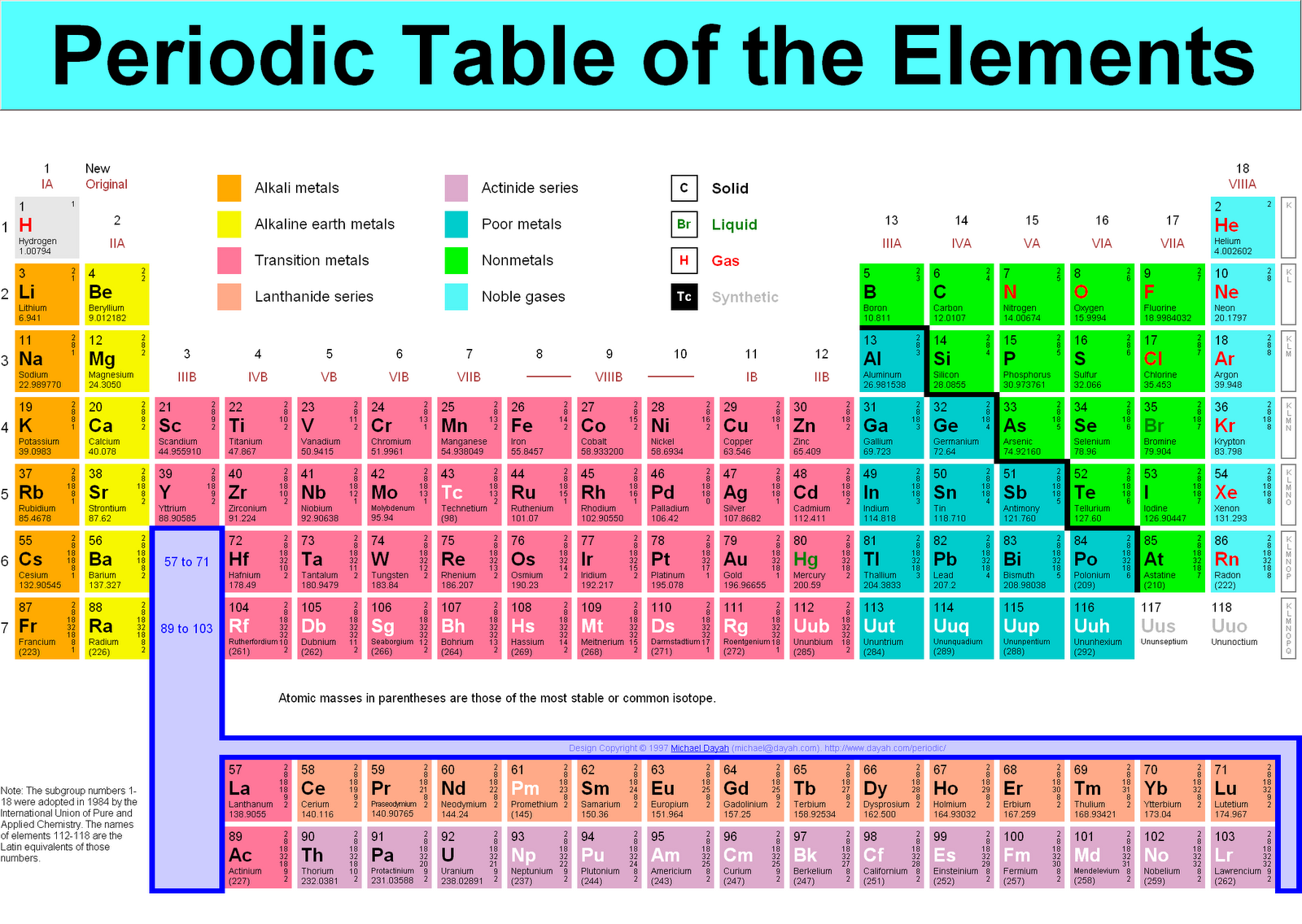
Image: everydayagri.blogspot.com
Rounding the atomic mass can simplify calculations and make it easier to compare the masses of different elements. It’s also a useful tool for understanding the periodic trends in atomic mass. For example, we can see that the atomic mass generally increases from left to right across a period and from top to bottom down a group. But there are some exceptions to this trend, which can be explained by the presence of isotopes and other factors.
Understanding Rounded Atomic Mass
What is rounded atomic mass?
Rounded atomic mass is a simplified representation of the average atomic mass of an element, rounded to the nearest whole number. It’s a convenient way to represent the mass of an element while still reflecting its relative position on the periodic table.
Why use rounded atomic mass?
There are several reasons why using rounded atomic mass can be beneficial:
- Simplicity: Rounded atomic mass makes calculations easier, especially for basic chemistry problems.
- Clarity: It provides a clear and quick way to compare the masses of different elements.
- Periodic trends: It highlights the general trends in atomic mass across the periodic table.

Image: studyonline.blog
The Importance of Isotopes
The concept of atomic mass is closely tied to the existence of isotopes. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in neutron count results in a slightly different atomic mass for each isotope. For example, carbon has two common isotopes, carbon-12 and carbon-14. Carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons. The atomic mass of carbon is listed as 12.011 amu (atomic mass units), which is a weighted average of the masses of its isotopes.
The relative abundance of each isotope within a sample of an element is crucial in determining its average atomic mass. This is why the atomic mass listed on the periodic table is a weighted average, taking into account the percentage of each isotope present in nature. However, for practical applications, rounding the atomic mass to the nearest whole number often provides sufficient accuracy.
Benefits of Using Rounded Atomic Mass
Using rounded atomic mass offers several advantages:
- Easier calculations: Rounding atomic mass simplifies calculations, especially when working with large numbers or complex formulas.
- Simplified comparisons: It facilitates easy comparison of elements based on their relative masses.
Interpreting Periodic Trends with Rounded Atomic Mass
Rounded atomic mass can be used to understand the general trends in atomic mass across the periodic table. For instance:
- Across a period (horizontal row): Atomic mass generally increases from left to right. This is because the number of protons and neutrons generally increases as you move across the period.
- Down a group (vertical column): Atomic mass generally increases from top to bottom. This is primarily due to the increasing number of electron shells, which leads to greater atomic size and therefore a larger atomic mass.
Examples of Rounded Atomic Mass in Practice
Let’s look at some examples of how rounded atomic mass is used:
- Calculating molecular mass: To calculate the molecular mass of a molecule, we typically use the rounded atomic masses of the elements involved.
- Balancing chemical equations: Rounding atomic mass can simplify the process of balancing chemical equations, especially when working with elements that have isotopes.
- Comparing elements: It can be used to easily compare the relative masses of different elements, which can be useful in deciding which element would be best suited for a particular application.
Tips for Using Rounded Atomic Mass
Keep in mind that using rounded atomic mass is not always appropriate. For very precise calculations or applications requiring high accuracy, it’s important to use the actual atomic mass listed on the periodic table.
FAQs about Rounded Atomic Mass
Q: Is it always okay to round atomic mass?
A: No, using rounded atomic mass is not always appropriate. For precise scientific work or applications requiring high accuracy, it’s essential to use the actual atomic mass from the periodic table.
Q: How do you round atomic mass?
A: You round the atomic mass to the nearest whole number. If the decimal portion is 0.5 or greater, round up. If it’s less than 0.5, round down.
Q: What is the difference between atomic mass and atomic number?
A: Atomic number refers to the number of protons in an atom’s nucleus, defining the element’s identity. Atomic mass, on the other hand, is the average mass of all the isotopes of that element, influenced by the number of protons and neutrons.
Periodic Table Of Elements With Rounded Atomic Mass
Conclusion
The rounded atomic mass is a valuable tool in chemistry, particularly in simplifying calculations and understanding periodic trends. It provides a quick and easy way to compare the relative masses of different elements, making it helpful in various applications. Understanding the difference between rounded and actual atomic mass is crucial, particularly when navigating precise scientific work.
Are you interested in learning more about the periodic table and its applications? If so, please share your thoughts and any questions you might have in the comments below.



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

