Have you ever wondered what makes a drink taste sour or what causes that burning sensation when you get soap in your eyes? The answers lie in the fascinating world of acids and bases, two fundamental concepts in chemistry that shape our everyday experiences. Understanding acids and bases is crucial for everything from baking and cleaning to the functioning of our bodies. Whether you’re a student grappling with these concepts or just curious about the chemistry that surrounds you, this guide will equip you with the knowledge and resources to confidently navigate the world of acids and bases.

Image: answermagictrommler.z19.web.core.windows.net
This article delves into the intriguing realm of acids and bases, providing detailed explanations, illustrative examples, and a comprehensive guide to successfully solving acids and bases worksheets. We’ll explore the history of these concepts, their essential properties, and their widespread applications in our daily lives. By the end, you’ll have a solid understanding of acids and bases, enabling you to tackle your coursework with confidence and approach everyday chemistry with a newfound appreciation.
The Fundamental Principles of Acids and Bases
What Exactly are Acids?
Acids are substances that release hydrogen ions (H+) when dissolved in water. These hydrogen ions are responsible for the characteristic sour taste of acids and their ability to react with metals, producing hydrogen gas. Common examples of acids include lemon juice, vinegar (acetic acid), and stomach acid (hydrochloric acid). The acidity of a substance is measured using the pH scale, with lower pH values indicating greater acidity.
Bases: The Opposites of Acids
Bases, on the other hand, are substances that release hydroxide ions (OH-) when dissolved in water. They have a slippery feel and a bitter taste. Bases are commonly known as alkalis. Sodium hydroxide (NaOH), also known as lye, and ammonia (NH3), found in common cleaning products, are examples of bases. Unlike acids, bases have a higher pH on the pH scale, with higher values indicating greater basicity.
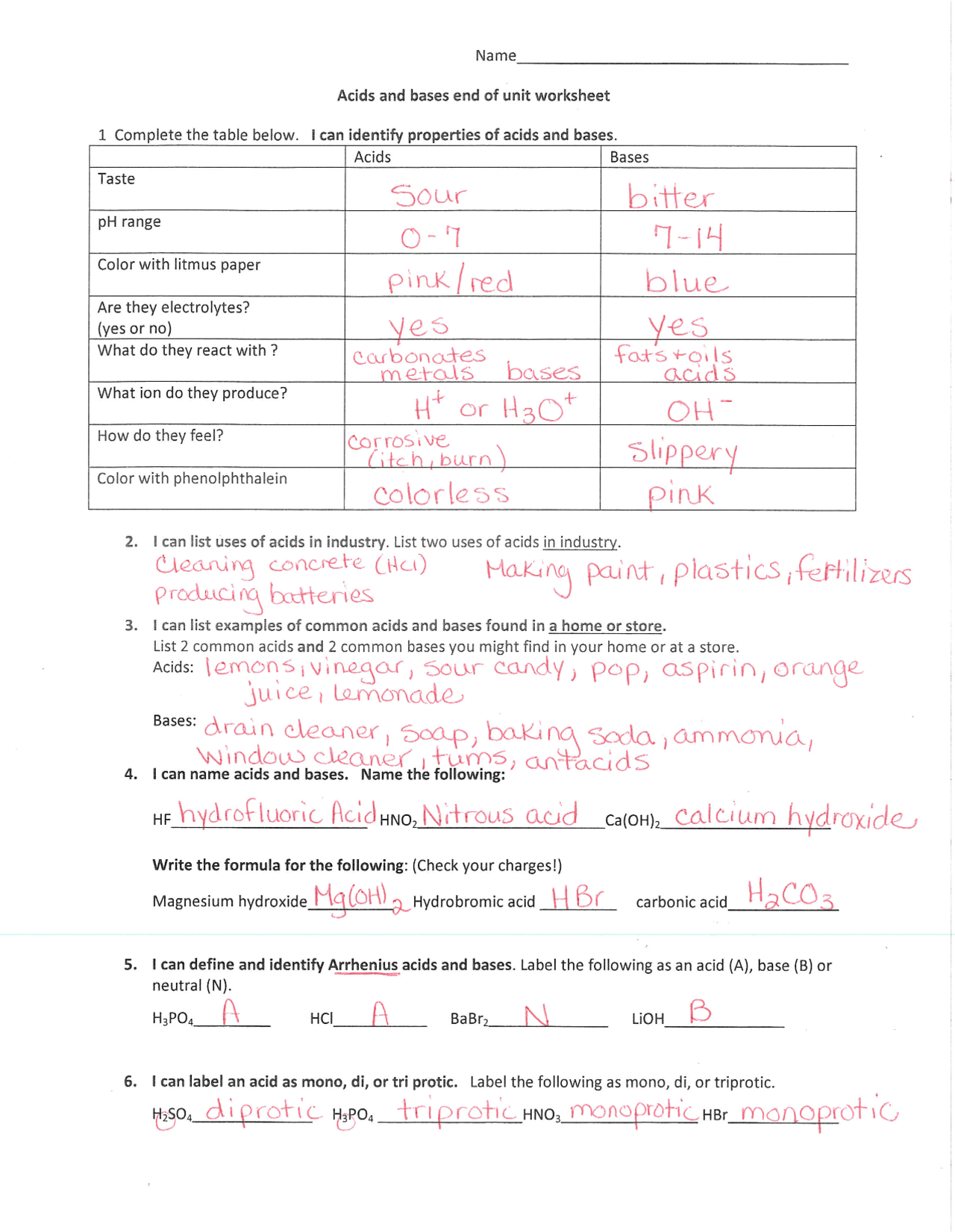
Image: classhofmann.z19.web.core.windows.net
pH Scale: Measuring Acidity and Basicity
The pH scale is a logarithmic scale that ranges from 0 to 14. A pH of 7 is neutral, indicating equal concentrations of H+ and OH- ions. Values below 7 indicate acidity, while values above 7 indicate basicity. The pH scale is crucial for understanding the chemical properties of substances and plays a vital role in various applications, such as the production of food, pharmaceuticals, and cleaning products.
The History of Acids and Bases
The understanding of acids and bases has evolved over centuries, with key contributions from renowned scientists, ultimately shaping our modern understanding of these fundamental concepts.
Early Observations: From Ancient Egypt to Medieval Alchemy
Early civilizations possessed rudimentary knowledge of acids and bases, using them in various applications. Ancient Egyptians, for example, used vinegar as a cleaning agent and for preserving food. Alchemists, who practiced a blend of chemistry, philosophy, and mystical practices in the Middle Ages, made significant advancements in understanding acids and bases. They experimented with various substances, discovering the corrosive nature of acids and the alkaline properties of bases.
Modern Foundations: The Contributions of Lavoisier and Arrhenius
In the late 18th century, the French chemist Antoine Lavoisier, considered the father of modern chemistry, proposed that acids contained oxygen. While his hypothesis was later proven incorrect, it paved the way for further investigations. Later, in the late 19th century, Swedish chemist Svante Arrhenius formulated his groundbreaking theory, which defined acids as substances that release hydrogen ions (H+) and bases as substances that release hydroxide ions (OH-) in an aqueous solution. Arrhenius’s theory formed the foundation of our modern understanding of acids and bases.
Real-World Applications of Acids and Bases
Acids and bases are not just confined to the laboratory; they are interwoven into our daily lives, playing crucial roles in diverse fields.
From Food and Beverages to Pharmaceuticals
The role of acids and bases in the food and beverage industry is undeniable. For example, citric acid, found in citrus fruits, adds a tangy flavor to various foods and drinks, while tartaric acid is used in baking powder. The pharmaceutical industry relies heavily on acids and bases for producing medicines. Aspirin, a common pain reliever, is a weak acid, while antacids, used to neutralize stomach acid, are typically basic compounds.
Essential Components in Cleaning Products
Acids and bases are key ingredients in many household cleaning products. Vinegar, a mild acid, is an effective cleaning agent, while baking soda, a base, is used for a variety of cleaning tasks, including removing stains and odors. Strong alkalis like sodium hydroxide and potassium hydroxide are used in drain cleaners, but handling these chemicals requires caution due to their corrosive nature.
Applying Your Knowledge: Acids and Bases Worksheets
Now that you have a solid understanding of acids and bases, you are ready to tackle those challenging worksheets. Here are some tips and strategies for successfully completing them:
Identify the Key Concepts: pH, Neutralization, and Indicators
Familiarize yourself with the key concepts that are tested in acids and bases worksheets, such as pH, neutralization reactions, and acid-base indicators. Understanding the pH scale and how to use it is crucial. Neutralization reactions involve the combination of an acid and a base to form salt and water. Acid-base indicators are substances that change color depending on the pH of a solution, allowing for a visual identification of acidity or basicity.
Practice with Examples: Master the Concepts
Work through practice problems and examples. Many online resources and textbooks offer a wealth of practice questions, which can help reinforce your understanding. For example, a typical worksheet might ask you to identify the pH of a particular solution or determine the products of a neutralization reaction. By practicing these exercises, you’ll gain confidence in applying the concepts to real-world situations.
Seek Clarification: Don’t Hesitate to Ask for Help
If you encounter difficulties, don’t hesitate to seek clarification. Consult your teacher, a tutor, or online resources. Online forums and communities dedicated to chemistry provide a platform for asking questions, sharing insights, and collaborating with other students. Remember, asking for help is a sign of strength, not weakness, and it can greatly enhance your understanding of the topic.
Acids And Bases Worksheet Answers Pdf
Conclusion: Embracing the World of Acids and Bases
By delving into the fascinating world of acids and bases, you have unlocked a deeper understanding of the chemical reactions that shape our world. From the tang of lemons to the cleaning power of vinegar and baking soda, acids and bases play a significant role in our daily lives. Armed with this knowledge, you can confidently tackle acids and bases worksheets and approach everyday chemistry with a newfound appreciation. Remember, learning is a continuous journey, and the more you explore, the more you’ll discover.



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

