Remember that moment in biology class when the teacher presented a demonstration involving a semipermeable membrane and two solutions of different concentrations? It felt like magic watching the water molecules move across the membrane, leaving us with a sense of awe and a burning desire to understand the science behind it. This curiosity led me on a journey into the fascinating world of diffusion and osmosis, two fundamental processes that govern the movement of molecules across membranes, and ultimately, the very life of organisms.

Image: www.studypool.com
While navigating the intricacies of diffusion and osmosis, I often found myself grappling with lab questions. The concepts seemed clear in theory, but applying them to practical scenarios presented unique challenges. Thankfully, with dedicated practice and a deeper understanding of the underlying principles, I began to unravel the mysteries of these processes. In this article, we’ll explore the fundamentals of diffusion and osmosis, delve into the key concepts that are essential for lab exercises, and offer practical tips to help you ace your next lab report.
Understanding the Principles of Diffusion and Osmosis
Diffusion: The Movement of Molecules Down a Concentration Gradient
Imagine dropping a single drop of food coloring into a glass of water. You’ll notice that the color gradually spreads throughout the water, eventually reaching a uniform distribution. This is because of diffusion, a passive process where molecules move from an area of high concentration to an area of low concentration. The driving force behind diffusion is the inherent tendency of molecules to maximize their entropy, or disorder, which leads them to spread out as evenly as possible.
In biological systems, diffusion plays a crucial role in the transport of essential nutrients, gases like oxygen and carbon dioxide, and waste products across cell membranes. The rate of diffusion is influenced by factors such as the concentration gradient, temperature, and the size and shape of the diffusing molecules.
Osmosis: The Special Case of Water Movement
Osmosis is a type of diffusion but with a specific focus on the movement of water molecules across a semipermeable membrane. This membrane allows water molecules to pass through while restricting the passage of larger molecules, such as solutes, effectively separating solutions of different concentrations.
The direction of water movement in osmosis is determined by the concentration of solutes on either side of the membrane. Water will always move from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration) to try and equalize the concentration across the membrane. This movement continues until an equilibrium is reached where the water potential on both sides is equal.
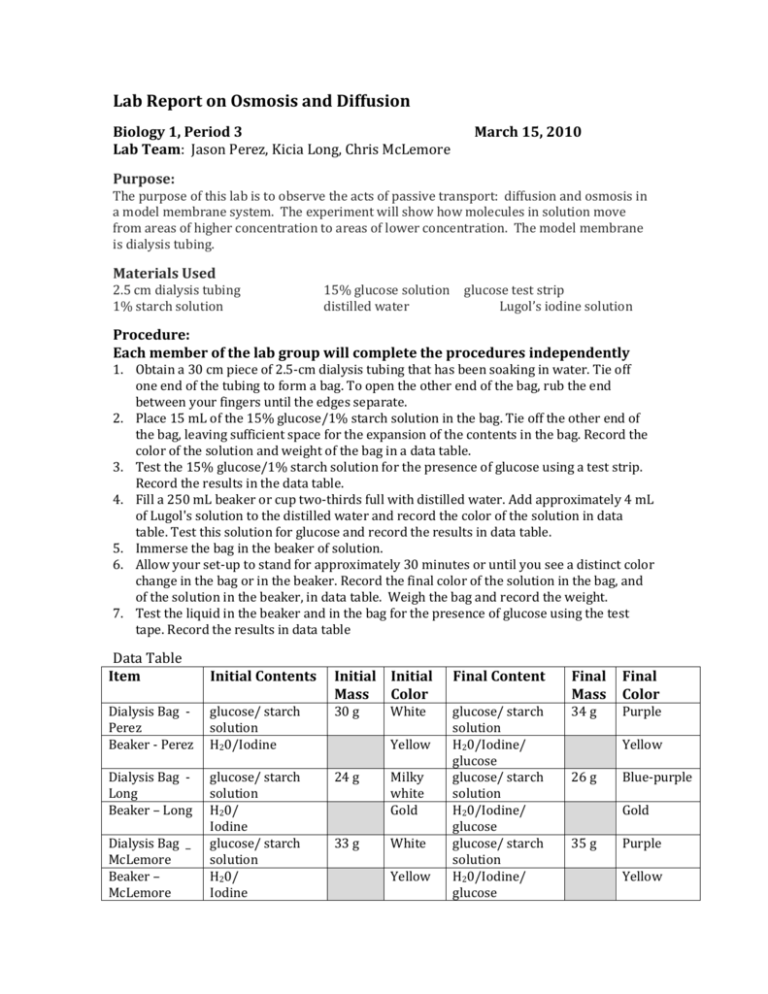
Image: www.vrogue.co
Lab Exercises: Unraveling the Concepts Through Experiments
Osmosis and diffusion are often explored in laboratory settings using practical demonstrations and experiments. These exercises provide a hands-on experience allowing students to observe the concepts in action and understand their implications.
For instance, a common experiment involves placing a potato core in a solution of varying concentrations. By observing the changes in the potato core’s weight and appearance, students can deduce the direction of water movement and the tonicity of the surrounding solution. The potato core will gain weight in a hypotonic solution, shrink in a hypertonic solution, and remain unchanged in an isotonic solution.
Other lab exercises might involve observing the diffusion of colored solutions across semipermeable membranes or studying the movement of red blood cells in solutions of varying solute concentrations. These experiments not only help visualize the principles of diffusion and osmosis but also provide a solid foundation for understanding their vital roles in maintaining cell function and homeostasis.
Key Factors Influencing Diffusion and Osmosis
Several factors contribute to the rate and direction of diffusion and osmosis, and understanding these influences is crucial for interpreting lab results and solving related questions.
Here are some of the key factors to consider:
- Concentration Gradient: The steeper the concentration gradient, the faster the rate of diffusion or osmosis. This is because the difference in concentration creates a greater driving force for molecules to move from the high concentration area to the low concentration area.
- Temperature: Higher temperatures lead to increased kinetic energy of molecules, resulting in faster diffusion and osmosis rates.
- Surface Area: A larger surface area exposed to diffusion allows for more molecules to cross the membrane simultaneously, increasing the rate of diffusion and osmosis.
- Permeability of the Membrane: The nature of the membrane plays a significant role. A highly permeable membrane allows for quicker movement of molecules, while a less permeable membrane restricts the flow.
- Molecular Size and Shape: Smaller molecules generally diffuse faster than larger ones, and molecules with simpler shapes often diffuse faster than those with complex structures.
By keeping these factors in mind while conducting lab experiments, you can effectively interpret the results and draw accurate conclusions about the process of diffusion and osmosis.
Tips and Tricks for Mastering Lab Answers
Here are some practical tips to help you excel in your diffusion and osmosis lab exercises:
- Thoroughly Review the Concepts: Before attempting any lab work, take the time to revisit the definitions, principles, and applications of diffusion and osmosis. Understanding the underlying science will make it easier to grasp the experimental setup and interpret the results.
- Pay Attention to Details: Lab exercises often involve specific instructions and requirements. Carefully read the instructions and ensure you understand the objective of each experiment.
- Maintain Accurate Record Keeping: Record your observations accurately and meticulously. This includes documenting the experimental setup, measurements taken, time intervals, and any changes observed. Accurate documentation will help you analyze the data and draw valid conclusions.
- Analyze Your Data: Don’t just report the observations; analyze the data to determine the relationships between the variables. For example, relate changes in the concentration gradient to the rate of diffusion or osmosis, or assess the effects of temperature or membrane permeability on the overall process.
- Connect Concepts to Real-World Applications: Diffusion and osmosis are not just theoretical concepts. They play crucial roles in biological processes, environmental phenomena, and technological applications. Relating these concepts to real-world examples will help deepen your understanding and make the learning process more engaging.
By following these tips and applying them diligently during your lab experiments, you’ll be well on your way to mastering the concepts of diffusion and osmosis and acing your lab reports.
Frequently Asked Questions (FAQ)
Q: What is the difference between diffusion and osmosis?
A: Diffusion refers to the general movement of molecules from an area of high concentration to an area of low concentration, driven by the tendency to maximize entropy. Osmosis is a specific type of diffusion that focuses exclusively on the movement of water molecules across a semipermeable membrane.
Q: Why is osmosis important in biological systems?
A: Osmosis plays a vital role in maintaining the fluid balance within cells and regulating the distribution of water throughout the body. It helps transport water across cell membranes, ensuring the proper hydration of cells and supporting various physiological processes.
Q: What is the relationship between osmosis and tonicity?
A: Tonicity refers to the relative solute concentration of two solutions separated by a semipermeable membrane. In a hypotonic solution, the solute concentration is lower than inside the cell, leading to water movement into the cell, causing it to swell or even lyse. In a hypertonic solution, the solute concentration is higher than inside the cell, leading to water movement out of the cell, causing it to shrink. An isotonic solution has equal solute concentrations on both sides of the membrane, resulting in no net water movement.
Q: How does temperature affect diffusion and osmosis?
A: Higher temperatures lead to an increase in the kinetic energy of molecules, resulting in faster rates of diffusion and osmosis. Warmer temperatures cause molecules to move faster and collide more frequently, accelerating the overall process.
One Diffusion And Osmosis Lab Answers
Conclusion
Mastering the concepts of diffusion and osmosis is essential for a deeper understanding of biological processes and their relevance in numerous applications. By understanding the fundamental principles, applying them to practical scenarios, and utilizing the tips and tricks outlined in this article, you can confidently tackle lab exercises and unlock the secrets of these fascinating phenomena.
Are you interested in learning more about diffusion and osmosis, or perhaps exploring specific applications in biology, chemistry, or even engineering? Let me know your thoughts in the comments below!



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

