Have you ever wondered why a feather and a brick, despite being vastly different in size, can be subject to the same force of gravity? Or how a ship, made of metal, can float on water? The answers lie in the fundamental concepts of mass, volume, and density. These three interconnected properties dictate how objects behave in the world around us. Understanding them unlocks a deeper understanding of physics and the world we inhabit.

Image: studylib.net
This article serves as your comprehensive guide to mastering mass, volume, and density. We’ll delve into the definitions of these terms, explore their interrelationships, and tackle practice problems to solidify your understanding. We’ll also provide answers to commonly encountered review worksheet questions, ensuring you’re equipped to tackle any challenge related to these key scientific concepts.
Understanding the Fundamentals: Mass, Volume, and Density
Mass: The Measure of Matter
Mass is a fundamental property of matter that quantifies the amount of substance present in an object. It essentially represents the resistance of an object to acceleration. In simpler terms, it’s how much “stuff” is in an object. We measure mass in kilograms (kg) in the International System of Units (SI). Imagine a large bag of flour – it has a significant mass due to the abundance of flour particles within it.
Volume: The Space Occupied
Volume, on the other hand, measures the three-dimensional space that an object occupies. It’s essentially how much room an object takes up. We measure volume in cubic meters (m3) in the SI system. Think of a large box: its volume is determined by the space enclosed within its six sides.
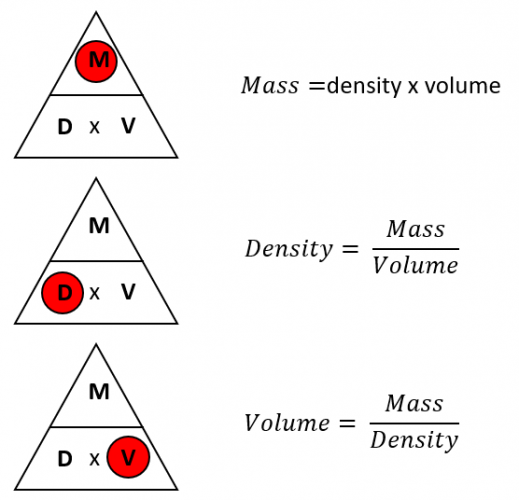
Image: www.tpsearchtool.com
Density: The Relationship between Mass and Volume
Density is the key that unlocks the relationship between mass and volume. It’s defined as the mass of an object per unit volume. In other words, density indicates how tightly packed the matter is within a given space. We measure density in kilograms per cubic meter (kg/m3) in the SI system. For example, a block of lead, with its tightly packed atoms, has a high density compared to a block of Styrofoam, which has a lower density.
Relationship Between Mass, Volume, and Density
The trio of mass, volume, and density is interconnected by the following equation:
Density = Mass / Volume
This simple equation reveals a crucial relationship: if we know any two of these quantities, we can easily calculate the third. For instance, if we have the mass and volume of an object, we can determine its density. This relationship is crucial for understanding how different substances behave, how objects float or sink, and how much force is needed to move them.
Practice Problems: Putting Concepts into Action
Let’s put our newfound knowledge to the test with some practice problems. These problems will help you apply the concepts learned and develop a deeper understanding of mass, volume, and density.
Example 1: Finding the Density of a Metal Block
Problem: A metal block has a mass of 5 kg and a volume of 0.2 m3. Calculate its density.
Solution:
Density = Mass / Volume
Density = 5 kg / 0.2 m3
Density = 25 kg/m3
Therefore, the density of the metal block is 25 kg/m3.
Example 2: Finding the Volume of a Liquid
Problem: A sample of water has a mass of 20 kg and a density of 1000 kg/m3. What is its volume?
Solution:
Density = Mass / Volume
Volume = Mass / Density
Volume = 20 kg / 1000 kg/m3
Volume = 0.02 m3
Therefore, the volume of the water sample is 0.02 m3.
Example 3: Finding the Mass of a Gas
Problem: A sample of air has a volume of 5 m3 and a density of 1.2 kg/m3. What is its mass?
Solution:
Density = Mass / Volume
Mass = Density x Volume
Mass = 1.2 kg/m3 x 5 m3
Mass = 6 kg
Therefore, the mass of the air sample is 6 kg.
Review Worksheet Answers: Solutions Explained
Now, let’s address common questions from review worksheets related to mass, volume, and density.
Question 1: What is the density of a substance with a mass of 10 g and a volume of 2 cm3?
Answer:
Density = Mass / Volume
Density = 10 g / 2 cm3
Density = 5 g/cm3
Question 2: A block of wood has a density of 0.8 g/cm3 and a volume of 50 cm3. What is its mass?
Answer:
Density = Mass / Volume
Mass = Density x Volume
Mass = 0.8 g/cm3 x 50 cm3
Mass = 40 g
Question 3: A metal sphere has a mass of 200 g and a density of 8 g/cm3. What is its volume?
Answer:
Density = Mass / Volume
Volume = Mass / Density
Volume = 200 g / 8 g/cm3
Volume = 25 cm3
Relating Concepts to Real-World Phenomena
The concepts of mass, volume, and density are not merely abstract theories. They play a crucial role in shaping our understanding of the world around us. Take, for example, the buoyancy of objects. Density determines whether an object sinks or floats in a fluid. A less dense object, like a boat, floats because it displaces more fluid than its weight. On the other hand, a denser object, like a rock, sinks because it displaces less fluid than its weight.
These fundamental concepts are utilized in various fields, including:
- Engineering: Civil engineers employ density calculations to determine the structural integrity of bridges and buildings.
- Materials Science: Researchers use density measurements to characterize and classify materials based on their composition and properties.
- Meteorology: Meteorologists use density measurements to understand atmospheric conditions and predict weather patterns.
Mass Volume And Density Practice Problems & Review Worksheet Answers
Conclusion: Embracing a Deeper Understanding of Matter
By understanding the concepts of mass, volume, and density, you unlock a gateway to comprehending the fundamental properties of matter and their impact on the world around us. Armed with this knowledge, you can tackle real-world problems with confidence, from designing sturdy structures to predicting weather patterns. Continue exploring these concepts, apply them in practice problems, and delve deeper into the fascinating realm of physics. Remember, the journey of learning is an ongoing endeavor, and every new concept learned opens doors to further exploration.



![Cyclomancy – The Secret of Psychic Power Control [PDF] Cyclomancy – The Secret of Psychic Power Control [PDF]](https://i3.wp.com/i.ebayimg.com/images/g/2OEAAOSwxehiulu5/s-l1600.jpg?w=740&resize=740,414&ssl=1)

